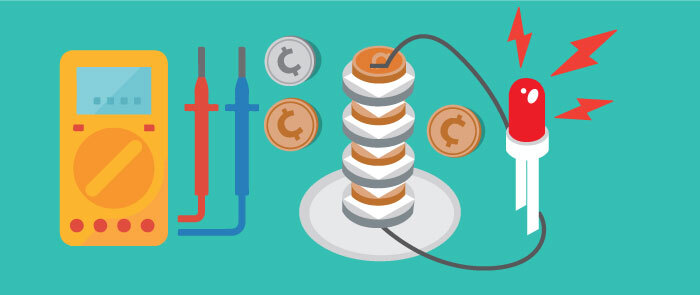
In this activity, you will use pennies and nickels to create a battery! This battery can even power an LED light.
Grade Levels: 4th grade and up
Time: 30 minutes
Materials:
- 6-8 pennies
- 6-8 nickels
- Strip of aluminum foil
- Paper towels or paper napkins
- ¼ cup white vinegar
- 1 tablespoon of salt
- Multimeter (voltage tester)
- Small LED pin light (optional)
- Small bowl
- Small plate
Steps:
- In a small bowl, combine the salt and vinegar and stir until the salt is dissolved.
- Cut the paper towels or paper napkins into small squares slightly smaller than the coins. You will need at least 20 squares.
- Dip the squares into the vinegar and salt mixture.
- Then build the battery. Place a dry paper towel on a plate. Take a strip of aluminum foil that’s about 1 inch x 3 inches and fold it lengthwise in thirds. Place the foil on the paper towel. Then layer the coins and the paper on top of the foil in a pattern: first a penny, then paper, then a nickel. Repeat the pattern until you run out of paper squares. The stack should have a penny on the bottom and a nickel on the top. The paper squares should not overlap or hang over the edge of the coins.
- Test the voltage of the battery. Touch the black lead to the strip of the aluminum foil and touch the red lead to the nickel on the top of the stack. Set the multimeter to a low voltage of direct current. Write down the reading shown on the multimeter.
- If you have an LED light, you can try attaching it to the battery. If the light doesn’t turn on, the battery may not have enough voltage. How do you think you can increase the voltage?
What is happening to make the battery work?
Nickels are made of a mixture of metals, including zinc. Pennies are made from several metals, including copper. Both zinc and copper conduct electricity. When two different metals are connected by an electrolyte (in this experiment, it’s the vinegar and salt solution), a chemical reaction occurs at the surface of the metals. The metals are the electrodes. When these electrodes are connected by a wire, they create an electrical current.
